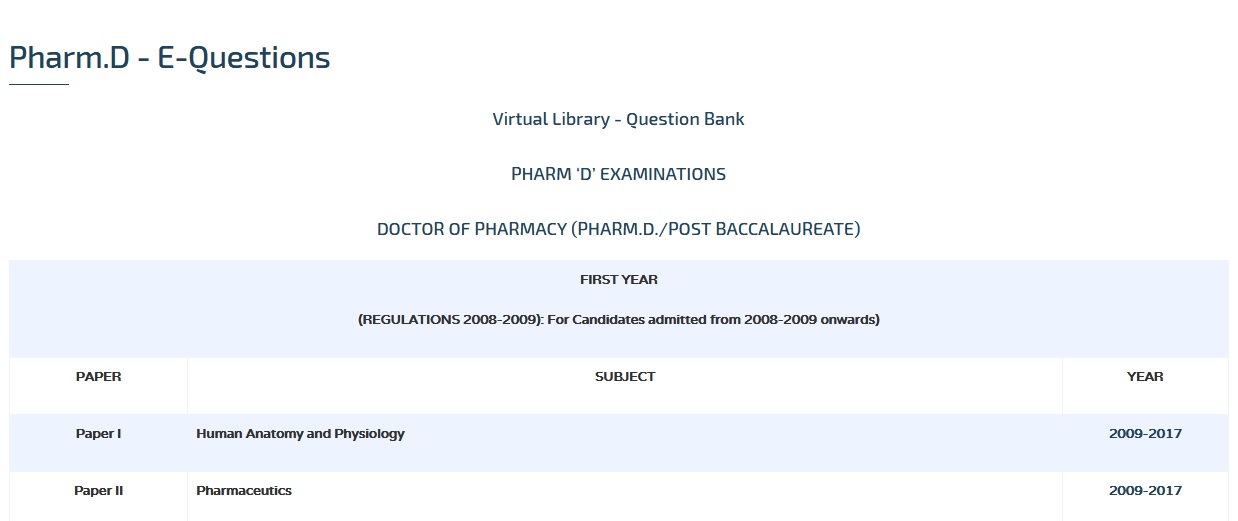
Pharmaceutical Jurisprudence D.Pharm Question Bank : web.tnmgrmu.ac.in
Name of the University : The Tamilnadu Dr. M.G.R. Medical University
Degree : D.Pharm
Subject Code/Name : 3816/Pharmaceutical Jurisprudence
Year : III
Paper : III
Document Type : Question Bank
Website : web.tnmgrmu.ac.in
Download Model/Sample Question Paper :
2011-2014 : https://www.pdfquestion.in/uploads/web.tnmgrmu.ac.in/PHARMACY/3811-383816KX.pdf
TNMGRMU Pharmaceutical Jurisprudence Questions
Sub. Code: 3816
PHARM. D DEGREE EXAMS
Related / Similar Question Bank :
TNMGRMU D.Pharm Pharmaceutical Formulations Question Bank
THIRD YEAR
PAPER IV – PHARMACEUTICAL JURISPRUDENCE

OCTOBER 2012
Q.P. Code : 383816
Time : 3 hours
Maximum : 100 marks
(180 Min)
Answer ALL questions in the same order. :
I. Elaborate on :
1. Discuss in detail about manufacture of alcoholic preparations in bonded laboratories.
2. Write about the salient features of drugs and magic remedies act and its rules.
II. Write notes on :
1. Explain about loan and repacking licence.
2. Write about the offences and penalities of narcotic and psychotropic substances act.
3. Discuss about Patents and Design act 1970.
4. What are the cautionary labels needed for the following drugs according to D & C act?
a. Schedule G b. Schedule H c. vertinary drugs
5. Discuss in brief about the Drug Technical Advisory Board and Drug consultative committee of Drugs and Cosmetics act.
6. Explain brief about sale of drugs under Drugs and Cosmetics act.
7. What are the Labelling requirements for the following drugs?
a. Alcoholic preparations
b. Colored preparations
c. Medicine for external use
8. Explain the Qualifications and duties of government analyst.
9. Write notes on non prescription drugs.
10.What are the ethics of pharmacist in relation to his trade?
APRIL 2013
I. Elaborate on : (2×20=40)
1. Explain about the objectives of pharmacy act and about constitutions and functions of Pharmacy Council of India.
2. Write in detail about the import of drugs and cosmetics act.
II. Write notes on : (10×6=60)
1. Write in short about warehousing of alcoholic preparations.
2. Explain briefly about cultivation, production and sale of opium under Narcotic and Psychotropic substances act.
3. What are the duties of a drug inspector?
4. What are the codes of ethics of a pharmacist in relation to his job?
5. Discuss in brief about Essential commodities act in relevant to Drug price control order.
6. Write about central drugs laboratories.
7. Write notes on National Drug Policy (current).
8. Explain about manufacture of drugs under schedule x drugs under Drugs and cosmetics act.
9. Write about import export and shipment of narcotic and psychotropic substances act.
10. What is the role of excise commission in alcoholic preparations premises under medicinal and toilet preparations act?
OCTOBER 2013
I. Elaborate on: (2 x 20 = 40)
1. What is the objective of Drugs and Cosmetics Act? Write a detail note on import of drugs under the act.
2. Give a detailed note on Patent and Drugs Act 1970.
II. Write notes on: (10 x 3 = 30)
1. What is schedule H, J and B in Drugs and Cosmetics act
2. Write the difference between manufacture of alcohol in bond and outside bond.
3. Pharmacist’s oath
4. Write the offences and penalties under Pharmacy Act.
5. Labeling requirements of Schedule G, H and X drugs
6. Warehousing of alcoholic preparations
7. Code of ethics for pharmacist in relation to his job
8. Loan license
9. Repacking license
10. Write the objectives of Excise Duties Act.
APRIL 2014
I. Elaborate on : (2×20=40)
1. Define patents. Write the provisions for getting the patents rights quoted in Patents Act.
2. Discuss in detail about manufacture of alcoholic preparations in bonded laboratories.
II. Write notes on : (10×3=30)
1. Write the role of pharmacist as a member of a health care team.
2. Joint State Pharmacy Council.
3. Outline about Repacking license.
4. Schedule J.
5. Write the functions of Drugs consultative committee.
6. Give an account on experimentation of animals under Prevention of Cruelty to Animals Act.
7. What are the sale conditions of Schedule H?
8. Give the details of narcotic drugs prescribed in Narcotic and Psychotropic Substances Act.
9. Salient features of New Drug Policy (current).
10.Mention the labeling requirements of Ayurvedic medicines.
October 2014
I. Elaborate on :
1. Give a brief note on various licenses required for the manufacture of drugs under Drugs and Cosmetics Act.
2. Give an account on the functions of Pharmacy Council of India constituted under the Pharmacy Act, 1948.
3. Discuss the manufacture of alcoholic preparations – Outside bond.
4. Discuss briefly about Drugs and Magic Remedies Act and Rules.
II. Write notes on :
1. Drug Technical Advisory Board as per Drugs and Cosmetic Act.
2. List out the patentable and non patentable inventions.
3. Draw a model label of Schedule H and X drugs including all the labeling requirements.
4. Experimentation on animals under Prevention of Cruelty to Animals Act
5. Drug Price Control order
6. Pharmaceutical Legislation in India
What are the difference between drug and medicine?
Drug is designed to produce specific reaction inside the body and medicine. Is there any substance designed to prevent or to treat disease?
Difference between bonded laboratory and non bonded laboratory.
Give the difference between bonded and non bonded laboratory